Synthesis of monosubstituted thioureas by vapour digestion and mechanochemical amination of thiocarbamoyl benzotriazoles†
Abstract
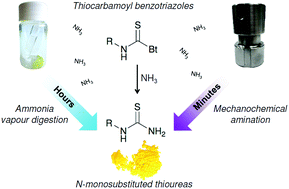
Thiocarbamoyl benzotriazoles, as safe and easy-to-handle isothiocyanate equivalents, were quantitatively converted to N-monosubstituted thioureas by vapour digestion synthesis under an ammonia atmosphere. This simple, but timely process provided a synthetic platform that enabled the “slow” amination reaction to be successfully transformed into a rapid one aided by mechanochemical milling. The ammonium chloride/sodium carbonate equimolar mixture allowed in situ formation of ammonia under ball-milling conditions. This novel and green approach yielded aromatic and aliphatic primary thioureas in near-quantitative isolated yields with workup entirely based on using only water. In addition, the molecular and crystal structures of selected polyaromatic primary thioureas were determined from the synchrotron powder diffraction data.

 Please wait while we load your content...
Please wait while we load your content...