An efficient synthesis of N-nitrosamines under solvent, metal and acid free conditions using tert-butyl nitrite†
Abstract
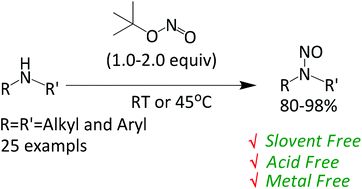
Synthesis of various N-nitroso compounds from secondary amines is reported using tert-butyl nitrite (TBN) under solvent free conditions. Broad substrate scope, metal and acid free conditions, easy isolation procedure and excellent yields are few important features of this methodology. The acid labile protecting groups such as tert-butyldimethylsilyl (TBDMS) and tert-butyloxycarbonyl (Boc) as well as sensitive functional groups such as phenols, olefins and alkynes are found to be stable under the standard reaction conditions. Besides N-nitrosation, TBN is also found to be an efficient reagent in few other transformations including aryl hydrazines to aryl azides and primary amides to carboxylic acids under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...