Synthesis of glycidyl azide polymers (GAPs) via binary ionic liquid–water mixtures without catalysts†
Abstract
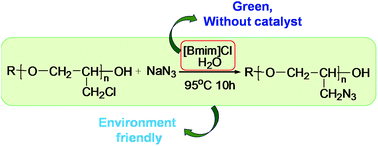
We report the preparation of glycidyl azide polymers (GAPs) by the reaction of a prepolymer polyepichlorohydrin (PECH) with sodium azide (NaN3) in mixture solvents of different mass ratios with ionic liquid 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) and water without catalysts. The formation of GAP was confirmed by IR and NMR spectroscopy, and the molecular weight of the product was determined by gel permeation chromatography (GPC). The conversion of PECH was identified via quantitative 13C-NMR spectroscopy. This method avoids solvent pollution and simplifies the reaction post-processing. The reaction was monitored by IR and 13C-NMR. We concluded that the relative solubility of the reaction substrate in the mixed solvents has an important effect on the degree of the reaction.

 Please wait while we load your content...
Please wait while we load your content...