Ligand-free Pd catalyzed cross-coupling reactions in an aqueous hydrotropic medium†
Abstract
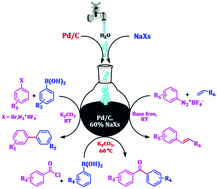
A simple, efficient and ligand-free protocol for the Suzuki–Miyaura reaction and base-free Heck–Matsuda reactions under mild reaction conditions have been developed over palladium supported on activated carbon (Pd/C) in an aqueous hydrotropic solution. The catalyst Pd/C was fully characterized by TG-DTA, SEM, EDS, XRD, XPS, BET and ICP-AES analyses. This green methodology represents a cost-effective and operationally convenient method for the synthesis of a variety of biaryls, prochiral ketones, and acrylates under the conditions that are tolerant for a broad range of functional groups with good to excellent yields. The developed Pd/C–aqueous hydrotrope combined catalytic system is well suited for the 3R approach (reducible, robust, and recyclable) for different cross-coupling reactions without an appreciable loss of its activity.

 Please wait while we load your content...
Please wait while we load your content...