Stereoselective organocatalysed reactions in deep eutectic solvents: highly tunable and biorenewable reaction media for sustainable organic synthesis†
Abstract
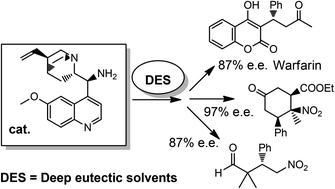
Three distinct stereoselective reactions, catalysed by using a chiral primary amine through different activation methods, have been successfully carried out for the first time in bio-based eutectic mixtures, thereby affording functionalised molecules in very high enantioselectivity. The use of these unconventional and biorenewable reaction media also provides opportunities for facilitating the recovery and the recycling of the chiral catalyst.

 Please wait while we load your content...
Please wait while we load your content...