Rusted iron wire waste into high performance anode (α-Fe2O3) for Li-ion batteries: an efficient waste management approach†
Abstract
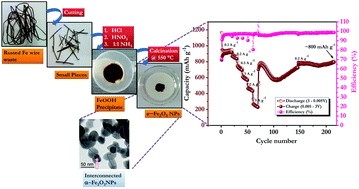
Superior Li-storage properties are reported for interconnected α-Fe2O3 derived from iron based wires collected from waste i.e. building supplies or scrap. An interconnected morphology is acquired without the addition of any surfactant or shape controlling agent. We also explore the possibility of employing such a material as a potential low cost conversion type anode for the fabrication of Li-ion cells with a LiMn2O4 cathode. Remarkably, α-Fe2O3 displayed a capacity of ∼1119 mA h g−1 at a current density of 0.05 A g−1 in a half-cell configuration. Good cyclability is also noted, for example α-Fe2O3 delivered ∼800 mA h g−1 after 215 cycles at a current density of 0.2 A g−1. The irreversible capacity loss of the α-Fe2O3 anode has been effectively circumvented by an electrochemical pre-lithiation process and the anode is eventually paired with the eco-friendly cathode, LiMn2O4. The full-cell, LiMn2O4/α-Fe2O3 delivered the initial reversible capacity of ∼737 mA h g−1 with ∼78% retention after 40 cycles. This efficient waste management system with a gram scale synthesis procedure for α-Fe2O3 nanoparticles indeed paved the way for developing high performance Li-ion power packs for high energy requirements.


 Please wait while we load your content...
Please wait while we load your content...