Selective reduction of nitroaromatics to azoxy compounds on supported Ag–Cu alloy nanoparticles through visible light irradiation†
Abstract
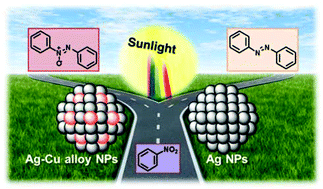
The selective hydrogenation of aromatic nitrocompounds to their corresponding azoxy compounds is challenging in organic synthesis, which are typically performed under harsh reaction conditions. The core issue involved in this reduction is to finely control the product selectivity. Herein, we report an efficient photocatalytic process using supported silver–copper alloy nanoparticles (Ag–Cu alloy NPs) to selectively transform nitrobenzene to azoxybenzene by visible light irradiation under green mild reaction conditions. Ag–Cu alloy NPs can absorb visible light, causing excited hot-electrons due to the localized surface plasmon resonance (LSPR) effect, and the excited electrons can activate the reactant molecule adsorbed on the NP surface to induce a reaction. The photocatalytic performance was affected by the Ag–Cu ratio, and the catalyst with an Ag–Cu molar ratio of 4–1 exhibited the optimal performance. Tuning the wavelength of incident light manipulated the product selectivity between azoxybenzene and aniline. Compared to pure Ag NPs, the alloying of Cu was found to be responsible for the product selectivity shifting from azobenzene to azoxybenzene. The reaction pathway was investigated to explain the selectivity difference and thus a tentative reaction pathway was proposed.

 Please wait while we load your content...
Please wait while we load your content...