Inhibition of α-glucosidase by vitamin D3 and the effect of vitamins B1 and B2
Abstract
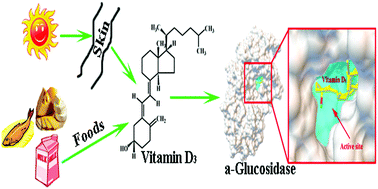
α-Glucosidase is a vital enzyme in carbohydrate metabolism. Over-expression of this enzyme is correlated with hyperglycemia. The inhibitory effect of vitamin D3 on α-glucosidase as well as its mechanism of action was investigated in this work. The results showed that vitamin D3 exhibited stronger inhibition on α-glucosidase than acarbose with the IC50 value of 1.28 × 10−4 mol L−1, and the inhibition was a mixed-type mechanism through a multiphase kinetic process. The inhibition constant was determined to be (5.66 ± 0.03) × 10−5 mol L−1. Vitamin D3 interacted with α-glucosidase by hydrophobic interactions, and molecular docking further verified that the inhibitor inserted into the active site pocket of α-glucosidase and interacted with the amino residues, which induced the rearrangement and conformational changes of α-glucosidase, and might move to cover the active pocket, hindering the binding of the substrate leading to the inhibition of the enzyme activity. Moreover, it was found that vitamin D3 combined with vitamin B1 or vitamin B2 exhibited significant synergistic effects on inhibition of α-glucosidase. This study has provided new insights into the role of vitamin D3 in inhibiting α-glucosidase catalysis and offered useful information on the dietary recommendation of vitamin D3 for the treatment of type 2 diabetes.
 Please wait while we load your content...
Please wait while we load your content...