Biotransformation of 8:2 fluorotelomer alcohol by recombinant human cytochrome P450s, human liver microsomes and human liver cytosol†
Abstract
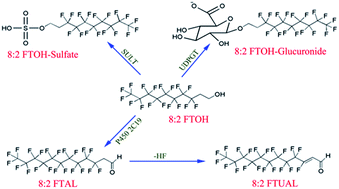
Biotransformation of 8:2 fluorotelomer alcohol (8:2 FTOH) can form potentially more toxic metabolites. However, the responsible cytochrome P450 (CYP) isoform(s) and phase II metabolism have not been studied in humans. Here, we characterized the in vitro metabolism of 8:2 FTOH by recombinant human CYPs, human liver microsomes, and human liver cytosol. The results showed that among the 11 isoforms investigated, CYP2C19 was the only enzyme capable of catalyzing 8:2 FTOH with Km and Vmax values of 18.8 μM and 8.52 pmol min−1 pmol−1 P450, respectively. The phase I metabolite was identified as 8:2 fluorotelomer aldehyde (8:2 FTAL). HLMs also catalyzed 8:2 FTOH transformation, with the Vmax and intrinsic clearance (CLint) values similar to those of CYP2C19 after the protein content is taken into account. Molecular docking showed that the hydroxyl group of 8:2 FTOH accesses the heme iron-oxo of CYP2C19 in an energetically favored orientation. 8:2 FTOH was also transformed by phase II enzymes to form O-glucuronide and O-sulfate conjugates. The CLint values follow the order of sulfation > oxidation > glucuronidation, suggesting that conjugation is the major metabolic pathway, which explains the low yield of perfluoroalkyl acids (PFCAs). These results provide new insight into fluorotelomer alcohol biotransformation and indirect human exposure to PFCAs.

 Please wait while we load your content...
Please wait while we load your content...