Activating earth-abundant electrocatalysts for efficient, low-cost hydrogen evolution/oxidation: sub-monolayer platinum coatings on titanium tungsten carbide nanoparticles†
Abstract
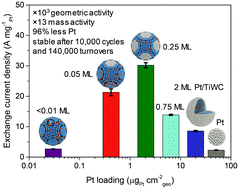
Most earth-abundant electrocatalysts suffer from negligible activity for the hydrogen oxidation reaction (HOR) and significant overpotentials for the hydrogen evolution reaction (HER) in acidic media. We designed earth-abundant, carbon-supported titanium tungsten carbide (TixW1−xC) nanoparticles decorated with surface Pt coatings ranging from the “single-atom” to the two-monolayer regime. Reactivity studies demonstrated that sub-monolayer Pt coverages are optimal and could activate the exposed metal carbide sites for both HER and HOR at low overpotentials. Specifically, a 0.25 monolayer coverage of Pt improved the exchange current density of Ti0.2W0.8C by more than three orders of magnitude. This catalyst outperformed traditional Pt/C by a factor of 13 on a Pt mass basis, allowing for over a 96% reduction in Pt loadings. Deactivation was not observed after 10 000 cycles between −50 and +600 mV vs. RHE in 1.0 M HClO4, and activity was maintained after 140 000 catalytic turnovers. A technoeconomic analysis revealed that over the catalyst lifetime, this new architecture could reduce materials and energy costs by a factor of 6 compared to state-of-the-art earth-abundant catalysts and a factor of 12 compared to Pt/C.

 Please wait while we load your content...
Please wait while we load your content...