Li–O2 cells with LiBr as an electrolyte and a redox mediator
Abstract
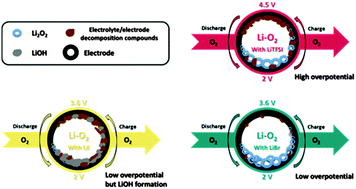
After many years of successful and disappointing results, the field of Li–O2 research seems to have reached an equilibrium state. The extensive knowledge that has accrued through advanced analytical studies enables us to delineate the weaknesses of the Li–O2 battery. It is now clear that the instability of the cell components toward extreme conditions existing during cell operation leads to early cell failure as well. One serious challenge is the high oxidation potential applied during the charge process. Redox-mediators may reduce the over-potential and, therefore, improve the efficiency and cyclability of Li–O2 cells. Their use in Li–O2 cells is mandatory. We have previously shown that LiI can indeed behave in such a manner; however, it also promotes the formation of side products during cell operation. We have, therefore, embarked on a comprehensive study of lithium halide salts as electrolytes for use in Li–O2 cells. We examine herein the effect of other components in the cell, such as solvents and contaminants, on the lithium halide salt activity. Based on the electrochemical behavior and the identity of the final cell products under various conditions, we can glean substantial information regarding the detailed operation mechanisms for each specific case. We have concluded that low concentration of LiBr in diglyme solution can improve the cell performance with fewer side effects than LiI. With LiBr, only the desired Li2O2 is formed during discharge. During charge, the bromine redox couple (Br−/Br3−) can reduce the oxidation potential to only 3.5 V. Higher efficiency and better cyclability of cells containing LiBr demonstrate that the electrolyte solution is the key to a successful Li–O2 battery.

 Please wait while we load your content...
Please wait while we load your content...