Increasing anti-cancer activity with longer tether lengths of group 9 Cp* complexes†
Abstract
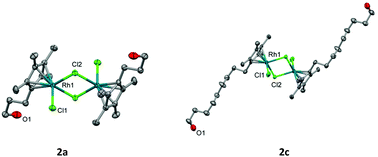
Here in, we report the cytotoxicity of both rhodium and iridium functionalised Cp* analogues of the [Cp*MCl2]2 dimers. The functionalised dimers contain a hydroxy tethered arm of differing carbon length. These show promising IC50 values when tested against HT-29, A2780 and cisplatin-resistant A2780cis human cancer cell lines, with the cytotoxicity improving proportionally with an increase in carbon tether length of the Cp* ring. The most promising results are seen for the 14-carbon Cp* tethered rhodium (2d) and iridium (3b) complexes, which show up to a 24-fold increase in IC50 compared to the unfunctionalised [Cp*MCl2]2 dimer. All complexes were potent inhibitors of purified thioredoxin reductase suggesting that disruption of cellular anti-oxidant function is one potential mechanism of action.

 Please wait while we load your content...
Please wait while we load your content...