Reductive silylation of Cp*UO2(MesPDIMe) promoted by Lewis bases†
Abstract
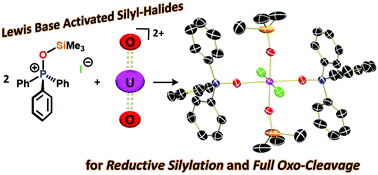
Functionalization of the uranyl moiety (UO22+) in Cp*UO2(MesPDIMe) (1-PDI) (MesPDIMe = 2,6-((Mes)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2C5H3N; Mes = 2,4,6-triphenylmethyl), which bears a reduced, monoanionic pyridine(diimine) ligand, is reported. Silylating reagents, R3Si-X (R = Me, X = Cl, I, OTf, SPh; R = Ph, X = Cl), effectively add across the strong O
CMe)2C5H3N; Mes = 2,4,6-triphenylmethyl), which bears a reduced, monoanionic pyridine(diimine) ligand, is reported. Silylating reagents, R3Si-X (R = Me, X = Cl, I, OTf, SPh; R = Ph, X = Cl), effectively add across the strong O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the presence of the Lewis base, OPPh3, generating products of the form (R3SiO)2UX2(OPPh3)2 (R = Me, X = I (2-OPPh3), Cl (3-OPPh3), SPh (5-OPPh3), OTf (6-OPPh3); R = Ph, X = Cl (4-OPPh3)). During this transformation, reduction to uranium(IV) occurs with loss of (Cp*)2 and MesPDIMe, each of which acts as a one-electron source. In the reaction, the Lewis base serves to activate the silyl halide, generating a more electrophilic silyl group, as determined by 29Si NMR spectroscopy, that undergoes facile transfer to the oxo groups. Complete U–O bond scission was accomplished by treating the uranium(IV) disiloxide compounds with additional silylating reagent, forming the family (Ph3PO)2UX4. All compounds were characterized by 1H NMR, infrared, and electronic absorption spectroscopies. X-ray crystallographic characterization was used to elucidate the structures of 2-OPPh3, 4-OPPh3, 5-OPPh3, and 6-OPPh3.
O bonds in the presence of the Lewis base, OPPh3, generating products of the form (R3SiO)2UX2(OPPh3)2 (R = Me, X = I (2-OPPh3), Cl (3-OPPh3), SPh (5-OPPh3), OTf (6-OPPh3); R = Ph, X = Cl (4-OPPh3)). During this transformation, reduction to uranium(IV) occurs with loss of (Cp*)2 and MesPDIMe, each of which acts as a one-electron source. In the reaction, the Lewis base serves to activate the silyl halide, generating a more electrophilic silyl group, as determined by 29Si NMR spectroscopy, that undergoes facile transfer to the oxo groups. Complete U–O bond scission was accomplished by treating the uranium(IV) disiloxide compounds with additional silylating reagent, forming the family (Ph3PO)2UX4. All compounds were characterized by 1H NMR, infrared, and electronic absorption spectroscopies. X-ray crystallographic characterization was used to elucidate the structures of 2-OPPh3, 4-OPPh3, 5-OPPh3, and 6-OPPh3.

 Please wait while we load your content...
Please wait while we load your content...