Synthesis and thermal stability of perovskite alkali metal strontium borohydrides†
Abstract
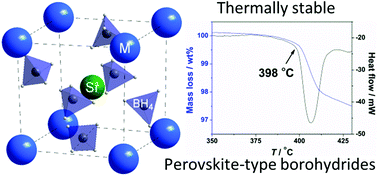
Three new perovskite-type bimetallic alkali metal strontium borohydride compounds, α-MSr(BH4)3 (M = K, Rb, Cs), have been synthesized and investigated by in situ synchrotron radiation powder X-ray diffraction, thermal analysis combined with mass spectrometry and Sievert's measurements. The bimetallic borohydrides were synthesized via an addition reaction between Sr(BH4)2 and MBH4 (M = K, Rb, Cs) by mechanochemical treatment. The Sr(BH4)2–NaBH4 system, which was treated in a similar manner, did not undergo reaction. All three α-MSr(BH4)3 compounds crystallize in the orthorhombic crystal system at room temperature: KSr(BH4)3 (P21cn), a = 7.8967(6), b = 8.2953(7), and c = 11.508(1) Å (V = 753.82(12) Å3). RbSr(BH4)3 (Pbn21), a = 8.0835(3), b = 8.3341(4), and c = 11.6600(5) Å (V = 785.52(6) Å3). CsSr(BH4)3 (P22121), a = 8.2068(9), b = 8.1793(9), and c = 6.0761(4) Å (V = 407.87(7) Å3). All three compounds are perovskite-type 3D framework structures built from distorted [Sr(BH4)6] octahedra. High-temperature polymorphs are identified to form at 258, 220 and 150 °C for MSr(BH4)3, M = K, Rb and Cs, respectively. The new compounds are thermally stable and decompose at T > 360 °C into SrB6, SrH2 and MBH4 (M = K, Rb, Cs).


 Please wait while we load your content...
Please wait while we load your content...