Calcium complexation and acid–base properties of l-gulonate, a diastereomer of d-gluconate†
Abstract
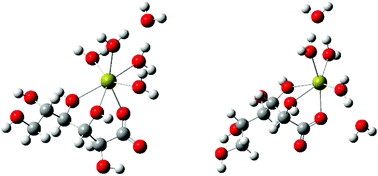
The Ca(II)-complexation and acid–base properties of L-gulonic acid (HGul), a diastereomer of D-gluconic acid (HGluc) differing only in the configurations of C2 and C5 have been investigated via1H and 13C NMR spectroscopies, Ca-ISE- and pH-potentiometry, polarimetry and freezing point depression. Data obtained for Gul−/HGul have been compared with those of Gluc−/HGluc. It was found that some properties (acid dissociation constant, the stoichiometry and formation constants of the Ca(II)-complexes) were insensitive to the difference in the configuration. In solutions with pH close to neutral, the presence of the complexes CaGul+ and CaGul20 was unambiguously proven, with formation constants of log K1,1 = 0.88 ± 0.02 and log β1,2 = 1.51 ± 0.03 (I = 1 M, T = 25 °C). The formation of Ca(Gluc)20 was also observed by others, which implies that the formation of the charge neutral 1 : 2 Ca(II)-complex of sugar carboxylates is more common than was previously believed. The stability of these species was found not to vary significantly in the ionic strength range of 1–4 M. Polarimetric measurements attested that the structure of Gul− did not change markedly upon complexation. NMR experiments suggest the coordination of C2-OH and C3-OH groups (beside COO−). DFT calculations support the existence of two coordination isomers, in which Ca2+ is attached to the COO−, C2-OH and C3-OH (in agreement with NMR), as well as to the COO−, C3-OH and C4-OH groups.

 Please wait while we load your content...
Please wait while we load your content...