Structure models for the hydrated and dehydrated nitrate-intercalated layered double hydroxide of Li and Al†
Abstract
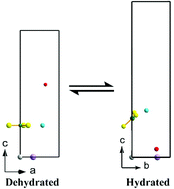
Imbibition of LiNO3 into gibbsite results in the formation of a single phase layered double hydroxide of the composition LiAl2(OH)6(NO3)·1.2H2O. This phase undergoes reversible dehydration along with the compression of the basal spacing accompanied by the reorientation of the nitrate in the interlayer gallery. The hydrated phase is a solid solution of two lattices: (i) a hexagonal lattice defining the ordering of atoms within the metal hydroxide layer, and (ii) a lattice of orthorhombic symmetry defining the ordering of atoms within the interlayer. DFT calculations of the hydration behaviour show that there is no registry between the two sublattices. In the dehydrated phase, the nitrate ion is intercalated with its molecular plane parallel to the metal hydroxide layer and the crystal adopts a structure of hexagonal symmetry.


 Please wait while we load your content...
Please wait while we load your content...