“Head-to-head” double-hamburger-like structure of di-ruthenated d(GpG) adducts of mono-functional Ru–arene anticancer complexes†
Abstract
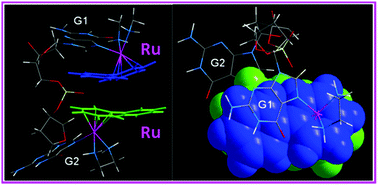
Guanine bases in DNA are targets for some Ru–arene anticancer complexes. We have investigated the structure of the novel di-ruthenated d(GpG) adduct Ru2-GpG (where Ru = {(η6-biphenyl)-Ru(en)}2+ (1′)) in aqueous solution. 2D NMR results indicate that there are two conformers, supported by modeling studies. The major conformer I is a novel double-hamburger-like structure with a “head-to-head” (HH) base arrangement involving hydrophobic interactions between neighboring arene rings, the first example of a HH d(GpG) adduct constructed by weak interactions. Hence there are significant differences compared to Pt-d(GpG) adducts formed by cisplatin. There is no obviously rigid bending for the major conformer I. The minor conformer II of Ru2-GpG has a back-to-back structure, with two ruthenated guanine bases flipped away from each other. 19-23 base-pair oligodeoxyribonucleotides containing central TGGT sequences di-ruthenated by 1 show no directional bending, only slightly distorted di-ruthenated duplexes, consistent with the NMR data for conformer I. The structural differences and similarities of d(GpG) residues which are di-ruthenated or cross-linked by platination are discussed in the context of the biological activity of these metal complexes.


 Please wait while we load your content...
Please wait while we load your content...