Characterization and utilization of Prussian blue and its pigments†
Abstract
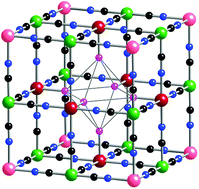
This review deals with our long-range goal of determining why the Prussian blue pigments, typically either the “soluble” KFeIII[FeII(CN)6]·xH2O or the alternative “insoluble” FeIII4[FeII(CN)6]3·xH2O compounds, used by artists from shortly after the discovery of Prussian blue in 1704 and well into the early twentieth century, often fade when exposed to light. In order to achieve this goal it was decided that first, for comparison purposes, we had to prepare and fully characterize Prussian blues prepared by various, often commercially successful, synthetic methods. The characterization has employed a large variety of modern methods to determine both the stoichiometry of the Prussian blues and the arrangement of the voids found in the latter “insoluble” Prussian blues. The refinement of synchrotron radiation derived X-ray powder diffraction data obtained for a formally soluble and an insoluble Prussian blue required refinement in the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and lead to the K1.9[FeIII4FeII3(CN)18]·{1.9 OH + 7.0H2O}, 1, and FeIII4FeII3(CN)18·11.0H2O, 2, stoichiometries. The former compound, 1, exhibits an apparently random iron(II) long-range void arrangement, whereas 2 exhibits a more non-random long-range arrangement, however, a pair distribution function analysis indicates a short-range ordering of the voids in both compounds. After further detailed characterization of many Prussian blue samples, painted samples on linen canvas, were subjected to accelerated light exposure for up to 800 hours either as pure Prussian blues or mixed with (PbCO3)2Pb(OH)2, ZnO or TiO2, the white pigments often used by artists to lighten the intense Prussian blue colour. The results indicate that the first two of these white pigments play a significant role in the fading of the colour of Prussian blues. In order to achieve our long-range goal, several Prussian blue samples were prepared from “ancient” recipes published in 1758 and 1779. These so-called “ancient” samples, painted in a dark and a pale blue shade, were also subjected to accelerated light exposure. The colorimetric results, in conjunction with X-ray powder diffraction refinements, pair distribution analysis and Mössbauer spectral results, indicate that, depending on the exact method of ancient preparation, the Prussian blue pigments were sometimes badly contaminated with alumina hydrate and/or ferrihydrite, a contamination which leads to extensive fading or decolourization of the Prussian blue pigments. The presence of ferrihydrite was subsequently confirmed in the study of a surface paint fragment from an eighteenth-century polychrome sculpture.
m space group and lead to the K1.9[FeIII4FeII3(CN)18]·{1.9 OH + 7.0H2O}, 1, and FeIII4FeII3(CN)18·11.0H2O, 2, stoichiometries. The former compound, 1, exhibits an apparently random iron(II) long-range void arrangement, whereas 2 exhibits a more non-random long-range arrangement, however, a pair distribution function analysis indicates a short-range ordering of the voids in both compounds. After further detailed characterization of many Prussian blue samples, painted samples on linen canvas, were subjected to accelerated light exposure for up to 800 hours either as pure Prussian blues or mixed with (PbCO3)2Pb(OH)2, ZnO or TiO2, the white pigments often used by artists to lighten the intense Prussian blue colour. The results indicate that the first two of these white pigments play a significant role in the fading of the colour of Prussian blues. In order to achieve our long-range goal, several Prussian blue samples were prepared from “ancient” recipes published in 1758 and 1779. These so-called “ancient” samples, painted in a dark and a pale blue shade, were also subjected to accelerated light exposure. The colorimetric results, in conjunction with X-ray powder diffraction refinements, pair distribution analysis and Mössbauer spectral results, indicate that, depending on the exact method of ancient preparation, the Prussian blue pigments were sometimes badly contaminated with alumina hydrate and/or ferrihydrite, a contamination which leads to extensive fading or decolourization of the Prussian blue pigments. The presence of ferrihydrite was subsequently confirmed in the study of a surface paint fragment from an eighteenth-century polychrome sculpture.

 Please wait while we load your content...
Please wait while we load your content...