A cooperative pathway for water activation using a bimetallic Pt0–CuI system†
Abstract
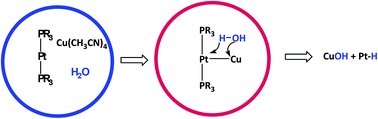
A mixture of the platinum(0) complex [Pt(PtBu3)2] and tetrakis(acetonitrile)copper(I) hexafluorophosphate in acetone activated a water molecule and gave the hydride platinum(II) complex [PtH(CH3CN)(PtBu3)2]PF6, 1, and the hydroxide Cu(I) species. The crystal structure of complex 1 was determined by X-ray crystallography, indicating a distorted square planar geometry around the platinum center. Although three possible mechanisms are proposed for this transformation, monitoring of the reaction using NMR spectroscopy at low temperature reveals that a cooperative pathway involving formation of a Pt0–CuI dative bond complex is the most probable pathway. The hydride platinum complex 1 is stable in acidic and neutral conditions but undergoes intramolecular C–H activation in the presence of pyridine. Monitoring of the reaction using 1H and 31P NMR spectroscopy shows that a cyclometalation reaction of one of the phosphine ligands is followed by displacement of a second phosphine ligand by pyridine to give the cyclometalated platinum(II) complex, [Pt(κ2PC-PtBu2CMe2CH2)(py)2], 4. The structure of 4 in solution and solid state phases was determined using NMR spectroscopy and X-ray crystallography, respectively.


 Please wait while we load your content...
Please wait while we load your content...