The selective activation of a C–F bond with an auxiliary strong Lewis acid: a method to change the activation preference of C–F and C–H bonds†
Abstract
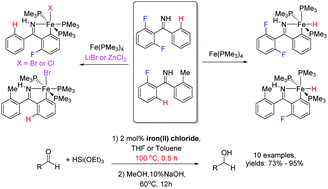
The selective activation of the C–F bonds in substituted (2,6-difluorophenyl)phenylimines (2,6-F2H3C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-n′-R-C6H4 (n′ = 2, R = H (1); n′ = 2, R = Me (2); n′ = 4, R = tBu (3))) by Fe(PMe3)4 with an auxiliary strong Lewis acid (LiBr, LiI, or ZnCl2) was explored. As a result, iron(II) halides ((H5C6-(C
NH)-n′-R-C6H4 (n′ = 2, R = H (1); n′ = 2, R = Me (2); n′ = 4, R = tBu (3))) by Fe(PMe3)4 with an auxiliary strong Lewis acid (LiBr, LiI, or ZnCl2) was explored. As a result, iron(II) halides ((H5C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-2-FH3C6)FeX(PMe3)3 (X = Br (8); Cl (9)) and (n-RH4C6-(C
NH)-2-FH3C6)FeX(PMe3)3 (X = Br (8); Cl (9)) and (n-RH4C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-2′-FH3C6)FeX(PMe3)3 (n = 2, R = Me, X = Br (11); n = 4, R = tBu, X = I (12))) were obtained. Under similar reaction conditions, using LiBF4 instead of LiBr or ZnCl2, the reaction of (2,6-difluorophenyl)phenylimine with Fe(PMe3)4 afforded an ionic complex [(2,6-F2H3C6-(C
NH)-2′-FH3C6)FeX(PMe3)3 (n = 2, R = Me, X = Br (11); n = 4, R = tBu, X = I (12))) were obtained. Under similar reaction conditions, using LiBF4 instead of LiBr or ZnCl2, the reaction of (2,6-difluorophenyl)phenylimine with Fe(PMe3)4 afforded an ionic complex [(2,6-F2H3C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-H4C6)Fe(PMe3)4](BF4) (10) via the activation of a C–H bond. The method of C–F bond activation with an auxiliary strong Lewis acid is appropriate for monofluoroarylmethanimines. Without the Lewis acid, iron(II) hydrides ((2-RH4C6-(C
NH)-H4C6)Fe(PMe3)4](BF4) (10) via the activation of a C–H bond. The method of C–F bond activation with an auxiliary strong Lewis acid is appropriate for monofluoroarylmethanimines. Without the Lewis acid, iron(II) hydrides ((2-RH4C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-2′-FH3C6)FeH(PMe3)3 (R = H (13); Me (14))) were generated from the reactions of Fe(PMe3)4 with the monofluoroarylmethanimines (2-FH4C6-(C
NH)-2′-FH3C6)FeH(PMe3)3 (R = H (13); Me (14))) were generated from the reactions of Fe(PMe3)4 with the monofluoroarylmethanimines (2-FH4C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-2′-RC6H4 (R = H (4); Me (5))); however, in the presence of ZnCl2 or LiBr, iron(II) halides ((2-RH4C6-(C
NH)-2′-RC6H4 (R = H (4); Me (5))); however, in the presence of ZnCl2 or LiBr, iron(II) halides ((2-RH4C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-H4C6)FeX(PMe3)3 (R = H, X = Cl (15); R = Me, X = Br (16))) could be obtained through the activation of a C–F bond. Furthermore, a C–F bond activation with good regioselectivity in (pentafluorophenyl)arylmethanimines (F5C6-(C
NH)-H4C6)FeX(PMe3)3 (R = H, X = Cl (15); R = Me, X = Br (16))) could be obtained through the activation of a C–F bond. Furthermore, a C–F bond activation with good regioselectivity in (pentafluorophenyl)arylmethanimines (F5C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-2,6-Y2C6H3 (Y = F (6); H (7))) could be realized in the presence of ZnCl2 to produce iron(II) chlorides ((2,6-Y2H3C6-(C
NH)-2,6-Y2C6H3 (Y = F (6); H (7))) could be realized in the presence of ZnCl2 to produce iron(II) chlorides ((2,6-Y2H3C6-(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)-F4C6)FeCl(PMe3)3 (Y = F (17); H (18))). This series of iron(II) halides could be used to catalyze the hydrosilylation reaction of aldehydes. Due to the stability of iron(II) halides to high temperature, the reaction mixture was allowed to be heated to 100 °C and the reaction could finish within 0.5 h.
NH)-F4C6)FeCl(PMe3)3 (Y = F (17); H (18))). This series of iron(II) halides could be used to catalyze the hydrosilylation reaction of aldehydes. Due to the stability of iron(II) halides to high temperature, the reaction mixture was allowed to be heated to 100 °C and the reaction could finish within 0.5 h.

 Please wait while we load your content...
Please wait while we load your content...