Hydroamination of carbodiimides, isocyanates, and isothiocyanates by a bis(phosphinoselenoic amide) supported titanium(iv) complex†
Abstract
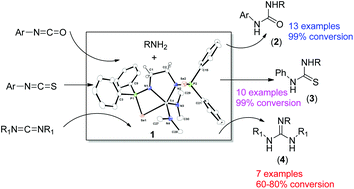
The hydroamination of heterocumulenes such as carbodiimides, isocyanates, and isothiocyanates by a bis(phosphinoselenoic amide) supported titanium(IV) complex as a precatalyst is reported here. The titanium(IV) complex [{Ph2P(Se)NCH2CH2NPPh2(Se)}Ti(NMe2)2] (1) was synthesised by the reaction of tetrakis-(dimethylamido)titanium(IV) [Ti(NMe2)4] with [{Ph2P(Se)NHCH2CH2NHPPh2(Se)}] in toluene at ambient temperature. Titanium complex 1 proved to be a competent pre-catalyst for the addition of an amine N–H bond to carbodiimides, isocyanates, and isothiocyanates. The reaction scope was expanded to reactions of aliphatic and aromatic amines with phenylisocyanates and phenylisothiocyanates in toluene solvents proceeding rapidly at room temperature with 5 mol% catalyst loadings to yield the corresponding urea and thio-urea derivatives up to 99%. However, ambient temperature was needed for hydroamination of 1,3-dicyclohexylcarbodiimide. The amine addition reactions with isocyanates showed first order kinetics with respect to catalyst 1 as well as substrates. The most plausible mechanism for the hydroamination reaction was established by isolating 1,1-dimethylphenyl urea as a side product.


 Please wait while we load your content...
Please wait while we load your content...