Intrinsic reactivity of a uranium metallacyclopropene toward unsaturated organic molecules†
Abstract
The uranium metallacyclopropene (η5-C5Me5)2U[η2-C2(SiMe3)2] (1) reacts with various small unsaturated organic molecules. For example, replacement of bis(trimethylsilyl)acetylene occurs when complex 1 is exposed to alkynes, conjugated alkenes, nitriles and quinones. Reaction of 1 with internal phenyl(alkyl)acetylene PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe selectively yields the Cs symmetric uranium metallacyclopentadiene (η5-C5Me5)2U[η2-C(Ph)
CMe selectively yields the Cs symmetric uranium metallacyclopentadiene (η5-C5Me5)2U[η2-C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)–C(Ph)
C(Me)–C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)] (6) after the loss of bis(trimethylsilyl)acetylene, while treatment of 1 with phenyl(silyl)acetylenes (PhC
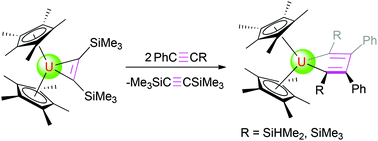
C(Me)] (6) after the loss of bis(trimethylsilyl)acetylene, while treatment of 1 with phenyl(silyl)acetylenes (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR, R = SiHMe2, SiMe3) gives the corresponding C2v symmetric isomers (η5-C5Me5)2U[η2-C(R)
CR, R = SiHMe2, SiMe3) gives the corresponding C2v symmetric isomers (η5-C5Me5)2U[η2-C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)–C(Ph)
C(Ph)–C(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)] (R = SiHMe2 (7), SiMe3 (8)). Furthermore, while no deprotonation occurs between complex 1 and pyridine derivatives, cyclohexanone can be inserted into the uranium metallacyclopropene moiety of 1 to yield the five-membered, heterocyclic complex (η5-C5Me5)2U[OC(CH2)5(C2(SiMe3)2)] (14) in quantitative conversion. Density functional theory (DFT) studies have been performed to complement the experimental studies.
C(R)] (R = SiHMe2 (7), SiMe3 (8)). Furthermore, while no deprotonation occurs between complex 1 and pyridine derivatives, cyclohexanone can be inserted into the uranium metallacyclopropene moiety of 1 to yield the five-membered, heterocyclic complex (η5-C5Me5)2U[OC(CH2)5(C2(SiMe3)2)] (14) in quantitative conversion. Density functional theory (DFT) studies have been performed to complement the experimental studies.

 Please wait while we load your content...
Please wait while we load your content...