Mono and dimetallic pyrene-imidazolylidene complexes of iridium(iii) for the deuteration of organic substrates and the C–C coupling of alcohols†
Abstract
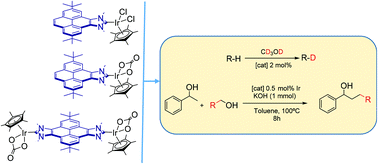
Three different Ir(III) complexes with pyrene-containing N-heterocyclic carbenes have been prepared and characterized. Two complexes contain a monodentate pyrene-imidazolylidene ligand, and have the formulae [IrCp*Cl2(pyrene-NHC)] and [IrCp*(CO3)(pyrene-NHC)]. The third complex is a dimetallic complex with a pyrene-di-imidazolylidene bridging ligand, with the formula [{IrCp*(CO3)}2(μ-pyrene-di-NHC)]. The catalytic activity of the three complexes was tested in the H/D exchange of organic substrates, and in the β-alkylation of 1-phenylethanol with primary alcohols. In the deuteration of organic substrates, the carbonate complexes are active even in the absence of additives. The dimetallic complex is the most active one in the catalytic coupling of alcohols, a result that may be interpreted as a consequence of the cooperativity between the two metal centres.

 Please wait while we load your content...
Please wait while we load your content...