Dialkylboron guanidinates: syntheses, structures and carbodiimide de-insertion reactions†
Abstract
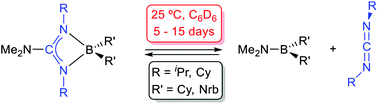
The synthesis of novel dialkylboron guanidinates is reported: the symmetrical compounds, (Me2N)C(NR)2BR′2 [R = iPr, R′ = Nrb (1); R = Cy, R′ = Nrb (2); R = iPr, R′ = Cy (3); R = R′ = Cy (4); R = 2,6-iPr2-C6H3; R′ = Cy (5); Nrb = exo-2-norbornyl] and the asymmetrically coordinated {iPr(H)N}C(NiPr)(NAr)BCy2 [Ar = Ph (6), 4-Me-C6H4 (7), 4-tBu-C6H4 (8)] were prepared by the salt metathesis method from the appropriate lithium guanidinates and chloroboranes. Moreover, the bis(dicyclohexylboron)guanidinate(−2) {iPr(Cy2B)N}C(NiPr){N(4-tBu-C6H4)}BCy2 (9) was also prepared from the corresponding dilithium guanidinate Li2[{N(4-tBu-C6H4)}C(NiPr)2] and ClBCy2. The structures of compounds 1, 3, 6 and 9 were confirmed by X-ray diffraction and all displayed a chelate coordination of the guanidinate ligand to the BR′2 fragment, the latter displaying an additional BCy2 attached to the exocyclic N atom. Solutions of compounds 1–4 reached an equilibrium with the aminoboranes Me2NBR′2 [R′ = Nrb (10), Cy (11)] and the corresponding carbodiimides, which was slow at 25 °C. The thermodynamic parameters for these equilibria are also reported. The activation parameters for the equilibrium for compound 1 have been calculated after a kinetic study. Compounds 5–8, with one or two N-aryl fragments bound to a B centre, are more robust and need higher temperatures (80 °C) and prolonged times to give similar carbodiimide de-insertion reactions.

 Please wait while we load your content...
Please wait while we load your content...