Three powerful dinuclear metal–organic catalysts for converting CO2 into organic carbonates†
Abstract
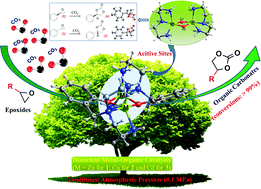
Developing efficient catalysts for converting carbon dioxide (CO2) into varied organic carbonates is an important scientific goal. By using the NH2-functionalized tripodal ligand 2-((bis(2-aminoethyl)amino)methyl)phenol (HL), three dinuclear metal–organic complexes [Zn(L)]2·2ClO4 (1), [Cu(L)]2·2ClO4·2H2O (2) and [Cd(L)]2·2ClO4 (3) have been successfully isolated and structurally characterized using single-crystal X-ray diffraction analyses. Considering the dinuclear metal centers and the NH2-functional groups in the structures, 1–3 were investigated as catalysts for converting CO2 into organic carbonates, and the results show that 1–3 exhibit an outstanding ability for converting CO2 into varied organic carbonates at atmospheric pressure (0.1 MPa). The catalytic system also displays a wide substrate scope and high catalytic activity, and the reaction mechanism has been proposed herein.

 Please wait while we load your content...
Please wait while we load your content...