Synthesis and isolation of non-chromophore cage-rearranged silsesquioxanes from base-catalyzed reactions†
Abstract
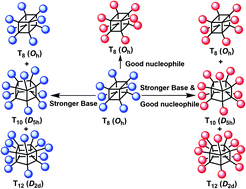
The nucleophilicity of both ortho- and meta-nitrophenolate anions is strong enough to give substituted products, but their basicity also facilitates cage-rearrangement reactions in polyhedral oligomeric silsesquioxanes (POSS). Anions having a stronger basicity, but weaker nucleophilicity, such as CO32−, gave products only from cage-rearrangement, with the cage expansion products being isolable in multi-gram quantities using conventional column chromatography.

 Please wait while we load your content...
Please wait while we load your content...