Syntheses and crystal structures of benzene-sulfonate and -carboxylate copper polymers and their application in the oxidation of cyclohexane in ionic liquid under mild conditions†
Abstract
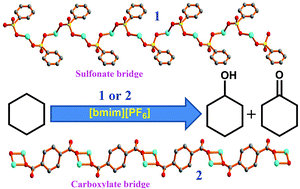
The syntheses, crystal structures and catalytic activities of the polymers derived from 2-(2-pyridylmethyleneamino)benzenesulfonic acid (HL), viz. [CuL(H2tma)]n (1) and [{Cu2L2(H2pma)}·(8H2O)]n (2) [H3tma = benzene-1,3,5-tricarboxylic (trimesic) acid and H4pma = benzene-1,2,4,5-tetracarboxylic (pyromellitic) acid], are presented. Despite the comparable combinations and compositions of ligands (sulfonate and carboxylate) in these two polymers the bridging moiety in 1 is sulfonate while in 2 it is carboxylate. Complexes 1 and 2 act as catalysts in the peroxidative oxidation of cyclohexane under mild conditions using either the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate [bmim][PF6] or acetonitrile as the solvent. The ionic liquid medium leads to increases in the yields and in the turnover numbers, achieved in shorter reaction times in comparison with those when using the conventional acetonitrile solvent. A simple recycling of the catalysts in the ionic liquid medium is achieved without loss of activity and selectivity.


 Please wait while we load your content...
Please wait while we load your content...