Two new Cu(ii) and La(iii) 2D coordination polymers, synthesis and in situ structural analysis by X-ray diffraction†
Abstract
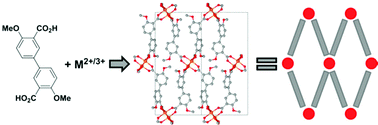
Two new coordination polymers were synthesized solvothermally using 4,4′-dimethoxy-3,3′-biphenyldicarboxylic acid (H2dmbpdc), and di- and trivalent metal salts (Cu(NO3)2·2.5H2O and La(NO3)3·6H2O). Their structures were determined by single-crystal X-ray diffraction analysis, and their thermal stability was evaluated by thermogravimetric analysis. The copper compound Cu(dmbpdc)(DMF; N,N-dimethylformamide), CPO-71-Cu, is based on the well known copper acetate paddlewheel secondary building unit. The asymmetric unit comprises one copper cation with one DMF molecule and one linker molecule coordinated. The lanthanum compound La2(dmbpdc)3(DMF)(H2O)3, CPO-72-La, is formed from a dimer of nine-coordinate, edge sharing lanthanum cations. To this dimer, three water molecules and one DMF molecule are coordinated in an ordered fashion. In addition, the asymmetric unit contains three crystallographically unique linker molecules. Both CPO-71-Cu and CPO-72-La form two-dimensional layered structures, and topological analyses reveal sql topologies with point symbol 44·62 and vertex symbol 4·4·4·4·6(2)·6(2). The thermal behavior of CPO-71-Cu was investigated in an in situ structural analysis by variable temperature powder- and single-crystal X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...