Discrimination of chiral copper(ii) complexes upon binding of galactonoamidine ligands†
Abstract
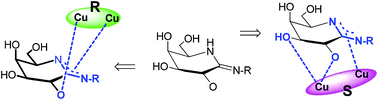
The coordination between N-p-methylbenzyl-D-galactonoamidine, a putative transition state analogue of the hydrolyis of glycosidic bonds, and symmetric and chiral binuclear copper(II) complexes was characterized by spectroscopic titration, isothermal titration calorimetry, circular dichroism spectroscopy, and DFT calculations to elucidate the binding sites in the carbohydrate upon coordination to selected metal complexes. For the formation of metal complex-glyconoamidine assemblies, contributions of the amidine site and of the hydroxyl group at C-2 in the glycon of the amidine are noted. The chiral complexes S- and R-Cu2bpdbo are discriminated by a third binding site in the carbohydrate that leads to higher stability of complexes derived from S-Cu2bpdbo (4–5 kcal mol−1) compared to those formed from R-Cu2bpdbo.

 Please wait while we load your content...
Please wait while we load your content...