Catalytic transfer hydrogenation of azobenzene by low-valent nickel complexes: a route to 1,2-disubstituted benzimidazoles and 2,4,5-trisubstituted imidazolines†
Abstract
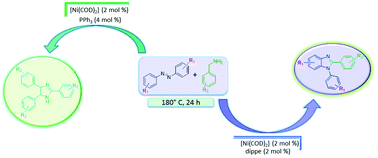
The one-pot synthesis of 1,2-disubstituted benzimidazoles by the transfer hydrogenation of azobenzene, using benzylamine as a hydrogen donor, sequential rearrangement of hydrazobenzene to semidine and further condensation with N-benzylideneamine is reported, catalyzed by 2 mol% of [Ni(COD)2] : dippe. The N2 substitution on benzimidazole can be controlled by the selection of different azobenzenes and C2 substitution will only depend on the chosen benzylamine. The current methodology avoids the addition of external oxidants, which are needed in the classical benzimidazole synthesis. In addition, the byproduct, N-benzylideneamine, obtained from dehydrogenation of benzylamine produced 2,4,5-trisubstituted imidazolines by cyclization and C–H functionalization, and this route was optimized with the use of 2 mol% of [Ni(COD)2] : 2PPh3.

 Please wait while we load your content...
Please wait while we load your content...