The use of organolithium reagents for the synthesis of 4-aryl-2-phenylpyridines and their corresponding iridium(iii) complexes†
Abstract
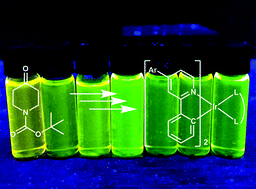
A versatile palladium-free route for the synthesis of 4-aryl-substituted phenylpyridines (ppy), starting from tert-butyl 4-oxopiperidine-1-carboxylate, is reported. Reaction with an aryllithium, followed by trifluoroacetic acid dehydration/deprotection and oxidation with 2-iodoylbenzoic acid and finally phenylation, gave 4 ligands (L1–4H): 2,4-diphenylpyridine, 4-(4-methoxyphenyl)-2-phenylpyridine, 2-phenyl-4-(o-tolyl)pyridine and 4-mesityl-2-phenylpyridine. These ligands were coordinated to iridium to give the corresponding Ir(L)2(A) complexes (Ir1–7), where A = ancillary ligand acetylacetate or 2-picolinate. This was used to demonstrate that, through a combination of ancillary ligand choice and torsional twisting between the 4-aryl substituents of the ppy ligands, it is possible to tune the phosphorescent emission of the complexes in the range 502–560 nm.

 Please wait while we load your content...
Please wait while we load your content...