Advances in the development of complexes that contain a group 13 element chalcogen multiple bond
Abstract
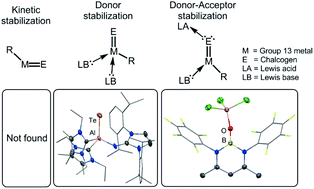
Inorganic group 13 element (M) chalcogenides (E) based on the general formular M2E3 are ubiquitous in synthesis, catalysis and material science. The parent ME fragment which aggregates to form three dimensional networks in the condensed phase can be expected to exhibit multiple bond character between the elements. Low temperature matrix isolation techniques are required to investigate the nature of this elusive species. An alternate approach for respective studies is the synthesis of electron-precise molecular complexes that contain the ME entity and for which isolation at ambient temperature is possible. This is realized by kinetic stabilization with bulky ligands and thermodynamic stabilization using electron donor, as well as acceptor groups attached to the ME functionality (i.e. donor–acceptor stabilization). In this article we revise the literature on complex compounds that exhibit a bonding interaction between a group 13 element atom and a chalcogen atom that is reasonably to be interpreted in terms of a double- or triple bond.

 Please wait while we load your content...
Please wait while we load your content...