Boron difluorides with formazanate ligands: redox-switchable fluorescent dyes with large stokes shifts†
Abstract
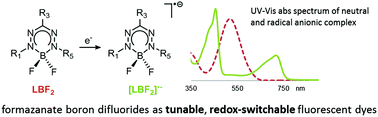
The synthesis of a series of (formazanate)boron difluorides and their 1-electron reduction products is described. The neutral compounds are fluorescent with large Stokes shifts. DFT calculations suggest that a large structural reorganization accompanies photoexictation and accounts for the large Stokes shift. Reduction of the neutral boron difluorides occurs at the ligand and generates the corresponding radical anions. These complexes are non-fluorescent, allowing switching of the emission by changing the ligand oxidation state.

 Please wait while we load your content...
Please wait while we load your content...