Insight into the reaction mechanisms for oxidative addition of strong σ bonds to an Al(i) center†
Abstract
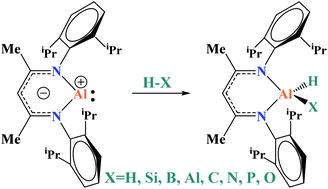
The oxidation addition of a series of σ H–X bonds (X = H, B, C, Si, N, P, and O) to a single Al(I) supported by a (NacNac)− bidentate ligand ((NacNac)− = [ArNC(Me)CHC(Me)NAr]− and Ar = 2,6-iPr2C6H3) has been explored through extensive DFT calculations. The presented results show that activation and addition of these σ bonds follow various reaction mechanisms, in which hydride transfer, proton transfer, and Al–X bond coupling steps are involved. The predicted free energy barriers for these oxidative additions range from 8 to 32 kcal mol−1, and all the reactions are remarkably favorable thermodynamically. However, sterically hindered ligands, for most reactants, make the formation of the initial reactant complex difficult and may reduce the efficiency of the reaction. Calculations reveal a strong dependence of the reaction mechanism and low-energy channel on the bonding features of X–H and the local structural environments.

 Please wait while we load your content...
Please wait while we load your content...