Capturing Re(i) in an neutral N,N,N pincer Scaffold and resulting enhanced absorption of visible light†
Abstract
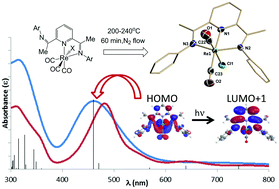
The organometallic and coordination chemistry of rhenium(I) has been largely restricted to bidentate α-diimine ligation and facial tricarbonyl coordination geometries. The thermal transformation of bidentate bis(imino)pyridine and bidentate terpyridine complexes at 200–240 °C under nitrogen led to a family of Re(I) pincer complexes [κ3-2,6-{ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}2(NC5H3)]Re(CO)2X (Ar
CMe}2(NC5H3)]Re(CO)2X (Ar![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H5, Me2C6H3, iPr2C6H3; X = Cl, Br) and (κ3-terpy)Re(CO)2X (X = Cl, Br). The synthesis, single crystal X-ray structural and spectroscopic characterization of these eight species documents their Re coordination geometries and demonstrates the accessibility of such compounds. The basic photophysical features of these compounds show significant elaboration in both number and intensity of the d–π* transitions observed in the UV-vis spectra relative to the bidentate starting materials and these spectra were analyzed using time-dependent DFT computations.
C6H5, Me2C6H3, iPr2C6H3; X = Cl, Br) and (κ3-terpy)Re(CO)2X (X = Cl, Br). The synthesis, single crystal X-ray structural and spectroscopic characterization of these eight species documents their Re coordination geometries and demonstrates the accessibility of such compounds. The basic photophysical features of these compounds show significant elaboration in both number and intensity of the d–π* transitions observed in the UV-vis spectra relative to the bidentate starting materials and these spectra were analyzed using time-dependent DFT computations.


 Please wait while we load your content...
Please wait while we load your content...