Multinuclear silver(i) XPhos complexes with cyclooctatetraene: photochemical C–C bond cleavage of acetonitrile and cyanide bridged Ag cluster formation†
Abstract
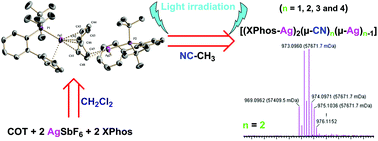
Cationic mono-, di-, tri- and tetra-nuclear silver complexes with Buchwald-type phosphane (XPhos) and cyclooctatetraene (COT) have been synthesized and characterized. Formation of [(XPhos-Ag)n(COT)][SbF6]n (n = 1 and 2) complexes was confirmed by single-crystal X-ray crystallography and multinuclear NMR spectroscopy. Variable-temperature NMR spectroscopy in CD2Cl2 solution shows the fluxionality of the COT ring in the mono-Ag(I) XPhos complex. Fluxionality of COT was also confirmed in the case of the di-Ag(I) XPhos complex by solid-state and solution 31P NMR spectroscopy. The C–C bond cleavage of coordinated acetonitrile [XPhos-Ag(I)-NCCH3] resulting in cyanide bridged Ag cluster formation [(XPhos-Ag)2(μ-CN)n(μ-Ag)n−1] (n = 1, 2, 3 and 4) upon light excitation of [(XPhos-Ag)n(COT)] was confirmed by HRESI-MS, UV-Absorption and HR-TEM.

 Please wait while we load your content...
Please wait while we load your content...