Quantifying the binding strength of salicylaldoxime–uranyl complexes relative to competing salicylaldoxime–transition metal ion complexes in aqueous solution: a combined experimental and computational study†
Abstract
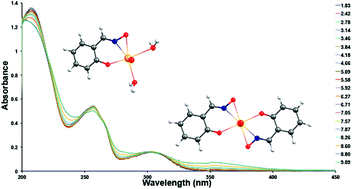
The design of new ligands and investigation of UO22+ complexations are an essential aspect of reducing the cost of extracting uranium from seawater, improving the sorption efficiency for uranium and the selectivity for uranium over competing ions (such as the transition metal cations). The binding strengths of salicylaldoxime–UO22+ complexes were quantified for the first time and compared with the binding strengths of salicylic acid–UO22+ and representative amidoxime–UO22+ complexes. We found that the binding strengths of salicylaldoxime–UO22+ complexes are ∼2–4 log β2 units greater in magnitude than their corresponding salicylic acid–UO22+ and representative amidoxime–UO22+ complexes; moreover, the selectivity of salicylaldoxime towards the UO22+ cation over competing Cu2+ and Fe3+ cations is far greater than those reported for salicylic acid and glutarimidedioxime in the literature. The higher UO22+ selectivity can likely be attributed to the different coordination modes observed for salicylaldoxime–UO22+ and salicylaldoxime–transition metal complexes. Density functional theory calculations indicate that salicylaldoxime can coordinate with UO22+ as a dianion species formed by η2 coordination of the aldoximate and monodentate binding of the phenolate group. In contrast, salicylaldoxime coordinates with transition metal cations as a monoanion species via a chelate formed between phenolate and the oxime N; the complexes are stabilized via hydrogen bonding interactions between the oxime OH group and phenolate. By coupling the experimentally determined thermodynamic constants and the results of theoretical computations, we are able to derive a number of ligand design principles to further improve the UO22+ cation affinity, and thus further increase the selectivity of salicylaldoxime derivatives.

 Please wait while we load your content...
Please wait while we load your content...