Structural analysis of ruthenium–arene complexes using ion mobility mass spectrometry, collision-induced dissociation, and DFT†
Abstract
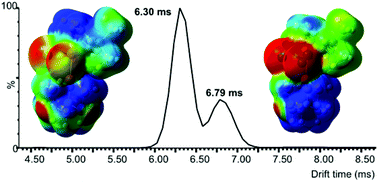
Ion mobility mass spectrometry (IM-MS) and collision-induced dissociation (CID) techniques were used to investigate the influence of the phosphine ligand on the physicochemical properties of [RuCl2(p-cymene)(PCy3)] (1), [RuCl2(p-cymene)(PPh3)] (2), and [RuCl2(p-cymene)(PTA)] (3) in the gas phase (PTA is 1,3,5-triaza-7-phosphaadamantane). Electrospray ionization of complexes 1 and 2 led to the corresponding [RuCl(p-cymene)(PR3)]+ ions via the dissociation of a chlorido ligand, whereas RAPTA-C (3) afforded two molecular ions by in-source oxidation ([RuIIICl2(p-cymene)(PTA)]+) or protonation ([RuCl2(p-cymene)(PTA+H)]+). Control experiments showed that the balance between these two ionization paths was strongly influenced by the nature of the solvent used for infusion. Collision cross sections (CCSs) of the four molecular ions accurately reflected the variations of steric bulk inferred from the Tolman steric parameters (θ) of the phosphine ligands. Moreover, DFT calculations combined with a model based on the kinetic theory of gases (the trajectory method of the IMoS software) afforded reliable CCS predictions. The almost two times higher dipole moment of [RuCl2(p-cymene)(PTA+H)]+ (μ = 13.75 D) compared to [RuIIICl2(p-cymene)(PTA)]+ (μ = 7.18 D) was held responsible for increased ion-induced dipole interactions with a polarizable drift gas such as N2. Further experiments with He and CO2 confirmed that increasing the polarizability of the buffer gas improved the separation between the two molecular ions derived from complex 3. The fragmentation patterns of complexes 1–3 were determined by CID. The sequence of collision voltages at which 50% of a precursor ion dissociates (V50) recorded for the molecular ions derived from compounds 1–3 was in good agreement with simple electronic considerations based on the donor strength of the phosphine ligand. Thus, the CCS and V50 parameters used to determine the shape and stability of ionic species in the gas phase are complementary to the Tolman steric and electronic parameters (θ and TEP) commonly used by organometallic chemists in condensed phases.

 Please wait while we load your content...
Please wait while we load your content...