Exploring the coordinative adaptation and molecular shapes of trinuclear Cu II2MII (M = Zn/Cd) complexes derived from salen type Schiff bases: structural and theoretical studies†
Abstract
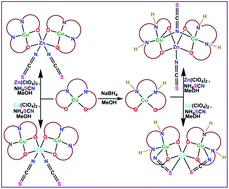
Three new trinuclear hetero-metallic complexes [(CuL)2Zn(NCS)2] (1), [(CuLR)2Zn(NCS)(μ1,1-NCS)] (2) and [(CuLR)2Cd(μ1,3-NCS)2] (4) have been synthesized using [CuL] and [CuLR] as “metalloligands” (where H2L = N,N′-bis(salicylidene)-1,3-propanediamine and H2LR = N,N′-bis(2-hydroxybenzyl)-1,3-propanediamine). All three complexes are characterized by elemental analysis, spectroscopic methods and single crystal XRD. Complex 1 is an angular trinuclear species, in which two terminal four-coordinate square planar “metalloligands” [CuL] are coordinated to a central Zn(II) through double phenoxido bridges along with two mutually cis nitrogen atoms of terminal isothiocyanate ions as is usually found in such complexes. In contrast, in complex 2, the two terminal “metalloligands” [CuLR] are square pyramidal, as one of the SCN− ions makes an unusual μ1,1-NCS bridge between copper centers while the other one coordinates to Zn(II) through a N atom in a usual fashion making its geometry also square pyramidal. For 4 which possesses an angular trinuclear structure, in addition to double phenoxido bridges from two terminal [CuLR], both the SCN− ions are S-bonded to Cd(II) and form a bridge (cis-μ1,3-SCN) between Cd(II) and each of the terminal Cu(II) ions. This structure is different from its unreduced analogue in which NCS− was N-terminal coordinated to Cd(II) (3/3′). All the structures have been optimized using density functional theory (DFT) calculations. It has been found that for H2L, optimized structures like 1 and 2 differ only by 0.4 kcal mol−1 but the H2LR structure 2 is more stable by 5.5 kcal mol−1 than the structure resembling 1. For Cd(II) complexes also, H2L optimized structures such as 3 and 4 do not differ significantly in energy (1.0 kcal mol−1) but the H2LR structure 4 is more stable than that of 3 by 4.6 kcal mol−1. In fact, structure 4 has been found to be the most stable one among the other possible isomers of H2LR.


 Please wait while we load your content...
Please wait while we load your content...