Beneficial effects of substituting trivalent ions in the B-site of La0.5Sr0.5Mn1−xAxO3 (A = Al, Ga, Sc) on the thermochemical generation of CO and H2 from CO2 and H2O†
Abstract
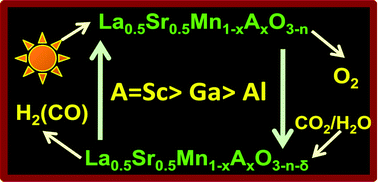
The effect of substitution of Al3+, Ga3+ and Sc3+ ions in the Mn3+ site of La0.5Sr0.5MnO3 on the thermochemical splitting of CO2 to generate CO has been studied in detail. Both La0.5Sr0.5Mn1−xGaxO3 and La0.5Sr0.5Mn1−xScxO3 give high yields of O2 and generate CO more efficiently than La0.5Sr0.5Mn1−xAlxO3 or the parent La0.5Sr0.5MnO3. Substitution of even 5% Sc3+ (x = 0.05) results in a remarkable improvement in performance. Thus La0.5Sr0.5Mn0.95Sc0.05O3 produces 417 μmol g−1 of O2 and 545 μmol g−1 of CO, respectively, i.e. 2 and 1.7 times more O2 and CO than La0.5Sr0.5MnO3. This manganite also generates H2 satisfactorily by the thermochemical splitting of H2O.

 Please wait while we load your content...
Please wait while we load your content...