Azide-bridged Cu(ii), Mn(ii) and Co(ii) coordination polymers constructed with a bifunctional ligand of 6-(1H-tetrazol-5-yl)-2,2′-bipyridine†
Abstract
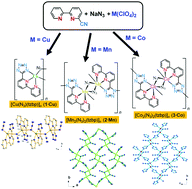
Three new azide-bridged coordination polymers, [M(N3)(tzbp)]n (M = Cu, 1·Cu; Mn, 2·Mn; Co, 3·Co), were successfully synthesized by the introduction of a bifunctional tetrazolate/2,2′-bipyridine ligand, 6-(1H-tetrazol-5-yl)-2,2′-bipyridine (Htzbp), from the in situ [2 + 3] cycloaddition of 6-cyano-2,2′-bipyridine in the presence of an excess of sodium azide under hydrothermal conditions. Compounds 1·Cu−3·Co were characterized by X-ray crystallography, IR spectroscopy, thermogravimetry, and elemental analysis. With tzbp− ligands acting in the chelating coordination mode, compound 1·Cu was comprised of a single end-on N3− (EO-N3) bridged one-dimensional (1D) zigzag structure. Both compounds 2·Mn and 3·Co adopt two-dimensional (2D) layered structures composed of a double EO-N3 bridged dinuclear motif, [M2(EO-N3)2], which are interlinked by tzbp− ligands in the chelating/bridging mode. The layers of 2·Mn and 3·Co are stacked on each other in ⋯ABAB⋯ and ⋯AAAA⋯ fashions, respectively. Magnetic investigations revealed that intrachain antiferromagnetic interactions were dominant in compound 1·Cu, and both 2·Mn and 3·Co exhibited spin-canted antiferromagnetism with critical temperatures (TN) of 3.0 and 18.4 K, respectively. Furthermore, below TN, the field-induced magnetic transitions of spin-flop and metamagnetism were observed in 2·Mn and 3·Co, respectively.

 Please wait while we load your content...
Please wait while we load your content...