Bovine serum albumin–cobalt(ii) Schiff base complex hybrid: an efficient artificial metalloenzyme for enantioselective sulfoxidation using hydrogen peroxide†
Abstract
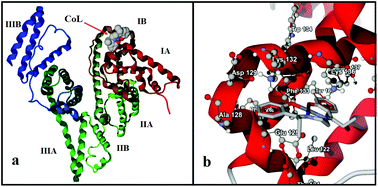
An artificial metalloenzyme (BSA–CoL) based on the incorporation of a cobalt(II) Schiff base complex {CoL, H2L = 2,2′-[(1,2-ethanediyl)bis(nitrilopropylidyne)]bisphenol} with bovine serum albumin (BSA) has been synthesized and characterized. Attention is focused on the catalytic activity of this artificial metalloenzyme for enantioselective oxidation of a variety of sulfides with H2O2. The influences of parameters such as pH, temperature, and the concentration of catalyst and oxidant on thioanisole as a model are investigated. Under optimum conditions, BSA–CoL as a hybrid biocatalyst is efficient for the enantioselective oxidation of a series of sulfides, producing the corresponding sulfoxides with excellent conversion (up to 100%), chemoselectivity (up to 100%) and good enantiomeric purity (up to 87% ee) in certain cases.

 Please wait while we load your content...
Please wait while we load your content...