Stabilisation of carbonyl free amidinato-manganese(ii) hydride complexes: “masked” sources of manganese(i) in organometallic synthesis†
Abstract
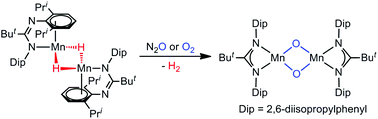
Reaction of the amidinato-manganese(II) bromide complex, [{(κ2-N,N′-Piso)Mn(μ-Br)}3(THF)2] (Piso = [(DipN)2CBut]−, Dip = 2,6-diisopropylphenyl), with K[BHEt3] affords the first example of a structurally authenticated amidinato-manganese(II) hydride complex, [{(N-,η3-arene-Piso)Mn(μ-H)2}2], via a process which involves a change in the amidinate coordination mode. Treatment of the bulkier precursor complex, [{(Piso′′)Mn(μ-Br)}n] (Piso′′ = [(Dip′′N)2CBut]−, Dip′′ = C6H2Pri2(CPh3)-2,6,4), with K[BHEt3] did not lead to an isolable manganese hydride complex, but its reaction with the magnesium(I) complex, [{(MesNacnac)Mg}2] (MesNacnac = [(MesNCMe)2CH]−, Mes = mesityl), did. This reaction presumably proceeds via a reactive manganese(I) intermediate, which abstracts hydrogen from a reaction component to give [{(κ2-N,N′-Piso′′)Mn(μ-H)}3]. A comparison of the reactivities of [{(N-,η3-arene-Piso)Mn(μ-H)2}2] and the isomorphous manganese(I) complex, [{(N-,η3-arene-Piso)Mn}2], toward CO, O2 and N2O was carried out. Reactions with the manganese(I) and manganese(II) species gave identical results, namely the formation of the manganese(I) carbonyl complex, [(κ2-N,N′-Piso)Mn(CO)4] (reactions with CO), and the manganese(III)-μ-oxo complex, [{(κ2-N,N′-Piso)Mn(μ-O)}2] (reactions with O2 and N2O). These results indicate that [{(N-,η3-arene-Piso)Mn(μ-H)2}2] can act as a “masked” source of an amidinato-manganese(I) fragment in synthetic transformations.

 Please wait while we load your content...
Please wait while we load your content...