Copper(ii) mixed-ligand polypyridyl complexes with doxycycline – structures and biological evaluation†
Abstract
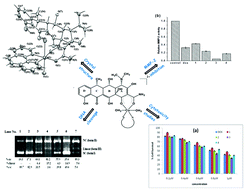
Mixed-ligand Cu(II) complexes of the type [Cu(doxycycline)(L)(H2O)2](NO3)2, where doxycycline = [4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide] and L = 2,2′-bipyridine (bpy, 1), 1,10-phenanthroline (phen, 2), dipyrido[3,2-d:2′,3′-f]quinoxaline (dpq, 3) and dipyrido[3,2-a:2′,3′-c]phenazine (dppz, 4) have been synthesised and characterised by structural, analytical, and spectral methods. The single-crystal X-ray structures of 1 and 2 exhibited two different geometries, distorted square-pyramidal and octahedral respectively as well as different coordination modes of doxycycline. Complexes 2–4 exhibit prominent plasmid DNA cleavage at significantly low concentrations probably by an oxidative mechanism. Matrix Metalloproteinase (MMP-2) inhibition studies revealed that all complexes inhibit MMP-2 similar to doxycycline which is a well-known MMP inhibitor with 3 being the most potent. IC50 values of doxycycline and 1–4 against MCF-7 (human breast cancer) and HeLa cell lines were almost equal in which 3 showed the highest efficiency (IC50 = 0.46 ± 0.05 μM), being consistent with its increased MMP inhibition potency. The antimalarial activities of these complexes against the chloroquine-sensitive Plasmodium falciparum NF54 and chloroquine-resistant Plasmodium falciparum Dd2 strains reveal that complex 3 exhibited a higher activity than artesunate drug against the chloroquine-resistant Dd2 strain.

 Please wait while we load your content...
Please wait while we load your content...