A mononuclear iron(ii) complex: cooperativity, kinetics and activation energy of the solvent-dependent spin transition†
Abstract
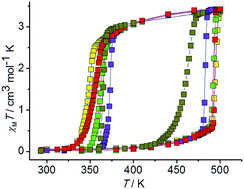
The system [FeL2](BF4)2 (1)–EtOH–H2O (L is 4-(3,5-dimethyl-1H-pyrazol-1-yl)-2-(pyridin-2-yl)-6-methylpyrimidine) shows a complicated balance between the relative stabilities of solvatomorphs and polymorphs of the complex [FeL2](BF4)2. New solvatomorphs, 1LS·EtOH·H2O and β-1LS·xH2O, were isolated in this system. They were converted into four daughter phases, 1A/LS, 1D/LS, 1E/LS·yEtOH·zH2O and 1F/LS. On thermal cycling in sealed ampoules, the phases 1LS·EtOH·H2O and β-1LS·xH2O transform into the anhydrous phase 1A/LS. The hysteresis loop width for the 1A/LS ↔ 1A/HS spin transition depends on the water and ethanol contents in the ampoule and varies from ca. 30 K up to 145 K. The reproducible hysteresis loop of 145 K is the widest ever reported one for a spin crossover complex. The phase 1A/LS combines the outstanding spin crossover properties with thermal robustness allowing for multiple cycling in sealed ampoules without degradation. The kinetics of the 1A/LS → 1A/HS transition is sigmoidal which is indicative of strong cooperative interactions. The cooperativity of the 1A/LS → 1A/HS transition is related to the formation of a 2D supramolecular structure of the phase 1A/LS. The activation energy for the spin transition is very high (hundreds of kJ mol−1). The kinetics of the 1A/HS → 1A/LS transition can either be sigmoidal or exponential depending on the water and ethanol contents in the ampoule. The phases 1D/LS and 1F/LS show gradual crossover, whereas the phase 1E/LS·yEtOH·yH2O shows a reversible hysteretic transition associated with the solvent molecule release and uptake.

 Please wait while we load your content...
Please wait while we load your content...