Mechanical mixtures of metal oxides and phosphorus pentoxide as novel precursors for the synthesis of transition-metal phosphides†
Abstract
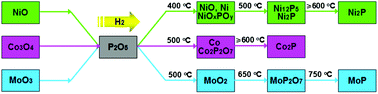
This study presents a new type of precursor, mechanical mixtures of metal oxides (MOs) and phosphorus pentoxide (P2O5) are used to synthesize Ni2P, Co2P and MoP phosphides by the H2 reduction method. In addition, this is first report of common solid-state P2O5 being used as a P source for the synthesis of metal phosphides. The traditional precursors are usually prepared via a complicated preparation procedure involving dissolution, drying and calcination steps. However, these novel MOs/P2O5 precursors can be obtained only by simple mechanical mixing of the starting materials. Furthermore, unlike the direct transformation from amorphous phases to phosphides, various specific intermediates were involved in the transformation from MOs/P2O5 to phosphides. It is worthy to note that the dispersions of Ni2P, Co2P and MoP obtained from MOs/P2O5 precursors were superior to those of the corresponding phosphides prepared from the abovementioned traditional precursors. It is suggested that the morphology of the as-prepared metal phosphides might be inherited from the corresponding MOs. Based on the results of XRD, XPS, SEM and TEM, the formation pathway of phosphides can be defined as MOs/P2O5 precursors → complex intermediates (metals, metal phosphates and metal oxide-phosphates) → metal phosphides.

 Please wait while we load your content...
Please wait while we load your content...