Synthesis and reactions of C-phosphanylated thiazol-2-thiones†
Abstract
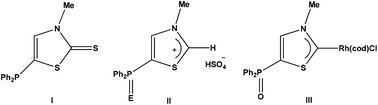
The facile regioselective synthesis of the P(III) substituted thiazol-2-thione 2 is presented. Reaction of 2 with hydrogenperoxide–urea, elemental sulfur and selenium resulted in P(V) chalcogenide thiazol-2-thiones 3–5. All compounds were characterized using 31P, 1H, 13C NMR, IR and elemental analyses and, additionally, by the single-crystal X-ray diffraction technique. Oxidative desulfurization of the 5-phosphinoylated thiazol-2-thione 3 using hydrogenperoxide led to the first C-phosphanoyl substituted thiazolium salt (6). Deprotonation of 6 and in situ reaction with the cyclooctadiene rhodium(I) chloride dimer yielded thiazol-2-ylidene rhodium(I) complex 7 which was confirmed by NMR spectroscopy and ESI-MS spectrometry.

 Please wait while we load your content...
Please wait while we load your content...