Synthesis and reactivity of α-cationic phosphines: the effect of imidazolinium and amidinium substituents†
Abstract
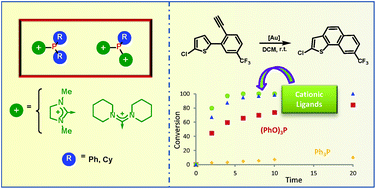
Mono- and dicationic phosphines have been synthesized through the reaction of chloroimidazolinium or chloroamidinium salts with secondary or primary phosphines respectively. The resulting ligands, which depict a significantly reduced donor ability compared with their neutral analogues, have been used to design Pt(II) and Au(I) complexes that effectively catalyse the hydroarylation of alkynes.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...