Al-free Fe-beta as a robust catalyst for selective reduction of nitric oxide by ammonia
Abstract
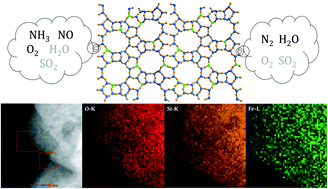
Al-free Fe-beta was prepared from commercial H-beta via a post-synthesis solid-state metallation route and applied as a promising catalyst for selective catalytic reduction of nitric oxide by ammonia. NH3-TPD and FTIR spectroscopy of pyridine adsorption analysis results clearly verify the absence of Brønsted acid sites and the formation of strong Lewis acid sites in the as-prepared Fe-beta zeolite. Isolated ferric ions within a zeolite framework are determined to be the dominating iron species in Fe-beta according to the characterization results from TEM, H2-TPR and UV-vis spectroscopy, and these Fe species are exposed and available for reaction as indicated by FTIR spectroscopy of NO adsorption. Fe-beta exhibited good catalytic activity in NH3-SCR reaction at >573 K, comparable with reference Fe–H-beta prepared via a solid-state ion exchange route, in the presence of SO2 and excess H2O. More importantly, Fe-beta exhibited distinctly better durability in NH3-SCR than reference Fe–H-beta, and a nitrogen oxide conversion of >90% at a reaction temperature of 673 K could be maintained for over 400 h. The remarkable durability of Fe-beta is due to the absence of framework aluminum species and the stabilization of active iron species by the zeolite framework. The reaction mechanism of NH3-SCR over an Fe-beta model catalyst with well-defined active iron sites was finally investigated by means of in situ DRIFT spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...