A highly-efficient La–MnOx catalyst for propane combustion: the promotional role of La and the effect of the preparation method
Abstract
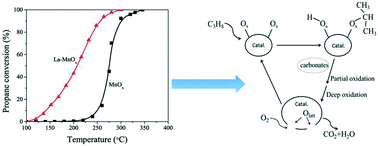
A La-modified MnOx catalyst was developed for the total oxidation of C3H8. The promotional role of La and the effect of the preparation method on the structural, physicochemical and catalytic properties of the La–MnOx catalyst were investigated. The results show that the presence of La in MnOx can remarkably improve the oxygen reactivity of MnOx and promotes the reduction properties of Mn species with the aid of a strong interaction between Mn and La. The preparation method obviously affected the crystal structure, lattice defects, surface phase composition, reduction properties and apparent morphology of the La0.4–MnOx catalyst. When the La0.4–MnOx (C-0.4) catalyst was prepared by using the co-precipitation method, it exhibited excellent catalytic performance, good thermal stability and enhanced H2O resistance to the title reation, because the C-0.4 sample possesses a higher atomic ratio of Mn4+/Mn3+ (2.03) and contains more absorbed oxygen species and high activity and mobility of lattice oxygen. In addition, the catalytic reaction mechanism of C3H8 oxidation was not changed by La doping but promoted the C3H8 adsorption on the catalyst surface, resulting in the acceleration of its rate-determining step to a large extent.

 Please wait while we load your content...
Please wait while we load your content...